Biochemist Rasa Petraitytė-Burneikienė: Reliability of Food Intolerance Blood Tests Debatable
Work in the laboratory. Associative photo by VU LSC junior researcher Arūnė Verbickaitė
A food intolerance blood test is designed to detect IgG antibodies against specific foods. However, our body produces these antibodies against the food we consume. It just happens naturally. Therefore, the results of food intolerance tests are questionable, says Vilnius University Life Sciences Center (VU LSC) researcher Dr. Rasa Petraitytė-Burneikienė, in whose laboratory the allergy test components are developed.
The researcher's group synthesizes virus-like particles in yeast, which can be applied in tests to detect various infectious diseases and in the development of vaccines.
Tell me, is developing tests to identify allergies difficult?
At the VU LSC laboratory, our focus is not on developing the tests themselves, but on synthesizing the key components for their development, recombinant allergens. These allergens are crucial in identifying various allergies, including food allergies (such as nuts and shrimp), house dust mite, dog and cat epithelial allergens, and bee and wasp venom allergies.
We insert allergen DNA into bacteria or yeast, and they produce allergens. Then, we purify those allergens: isolate them from bacteria or yeast cells, characterize them, and evaluate whether they are suitable for diagnostic purposes.
Since we cooperate with companies that develop tests and consult, and we are interested in how tests are developed, I can tell you in more detail. Test development is a complex process; many different variations need to be tested for the test to be reliable.
Allergy diagnostic tests are blood tests that determine if the immune system has produced IgE class antibodies in response to specific allergens. If the amount of these IgE antibodies in the blood to a particular allergen is high, then the person is allergic to that substance.
When developing tests, it is first necessary to identify the allergy-causing molecule of a specific protein. Once it is determined, test development, optimization of its performance, and assessment of accuracy and reliability begin. Each allergenic molecule has certain properties that can affect the test development process, so it is necessary to consider the physicochemical properties of that molecule. In addition, it is crucial to evaluate the sensitivity and specificity of tests to identify allergies as accurately as possible and avoid or reduce false positives or negatives.
How accurate are skin prick allergy tests?
The skin prick test is very popular, quick, inexpensive, and accurate. However, it sometimes produces false positive or false negative results.
False positive results occur when the test shows a reaction to an allergen to which a person is not sensitive. This can happen for several reasons. For example, when studying food allergies, inaccuracies can occur in the tests because our body breaks down proteins during digestion, and IgE antibodies are not produced against those smaller fragments. The food proteins used in skin prick tests are larger (natural proteins), so the test may show a positive reaction - that the sensitivity to a particular food is higher than it really is.
Another reason for a false positive result is cross-reactivity. For example, peanuts are legumes, including beans, peas, and lentils. The test may show a reaction to beans when, in fact, a person may only have an allergy to peanuts. It happens because these legumes have a protein similar to the one detected by the test. Most people with peanut allergies can eat other legumes without experiencing any allergy symptoms.
False negative results can be obtained when the test does not detect an allergy, and a person feels allergy symptoms. In this case, it may be necessary to carry out additional molecular tests, which are performed using blood samples and detect IgE antibodies specific to a particular allergen.
In addition, skin prick tests may fail due to the type of allergen extract used. Allergen extracts are protein mixtures prepared from, for example, certain foods, dust mites, pet epithelial cells, or plant pollen. The amount of protein in the extracts can vary significantly because the extract may not contain the allergenic protein at all, so we will not detect an allergy. Therefore, it may be necessary to conduct additional tests, such as a blood test, to check for IgE antibodies to ensure the accuracy of the results. Recombinant allergen proteins are also required to detect specific IgE in blood tests - these are the ones we create in our laboratory.
You’ve mentioned a blood test to detect IgE antibodies. Are these tests more accurate than the skin prick test?
Increased IgE antibody level in the blood indicates patient sensitization. It is possible to test sensitization to several hundred different allergens during one test. This test is handy if you have multiple allergies. It can provide quantitative measurements of IgE antibody levels, which allow an assessment of the severity of allergic sensitization.
However, false negative or false positive results also occur here. Elevated IgE levels alone do not necessarily indicate an allergy if you do not experience any allergy symptoms. This is called asymptomatic sensitization.
In the case of asymptomatic sensitization, the human body produces IgE antibodies in response to the allergen. However, when exposed to that allergen, it has no allergy symptoms because the body has mechanisms to regulate the immune response. These mechanisms can sometimes suppress allergic reactions, even in case of elevated IgE levels. In addition, allergic reactions occur when the immune response exceeds a certain threshold.
Only an allergist who evaluates your condition and the blood test results can draw conclusions about the allergies identified.
Blood tests for food intolerance are often offered in clinics, especially private ones. What do you think about them?
The utility and reliability of these tests are questionable, as IgG antibodies form as a natural immune response of the organism to antigens present in the food. The presence of IgG antibodies indicates that the body has been exposed to certain foods, and this is not an unwanted, side reaction or intolerance to that food, thus IgG antibodies are not considered reliable markers for identifying food allergies, intolerances, or sensitivities. The body produces IgG antibodies against the food we have eaten. It just happens naturally. Therefore, IgG's determination in the food intolerance assessment is controversial. I’d like to point out that I'm not talking about the tests that detect digestive enzyme deficiencies. Deficiency of digestive enzymes is the true food intolerance, and it is determined not by measuring IgG at all, but by other methods, e.g., genetic, PCR tests.
Another of your scientific interests is the synthesis of virus-like particles in yeast. How does this synthesis take place? How do such particles differ from real viruses?
Synthesis of virus-like particles in yeast is carried out by recombinant protein technology. To explain briefly, it happens when the genes encoding viral proteins are inserted into yeast cells. The virus proteins synthesized in yeast self-assemble to form structures similar to viral particles. The virus-like particles are released from the yeast cells and used for various purposes.
These particles cannot reproduce and cause infection. Although they are similar in structure to real viruses, they lack the genetic material (DNA or RNA) needed for the virus to reproduce. Therefore, they do not pose any danger.
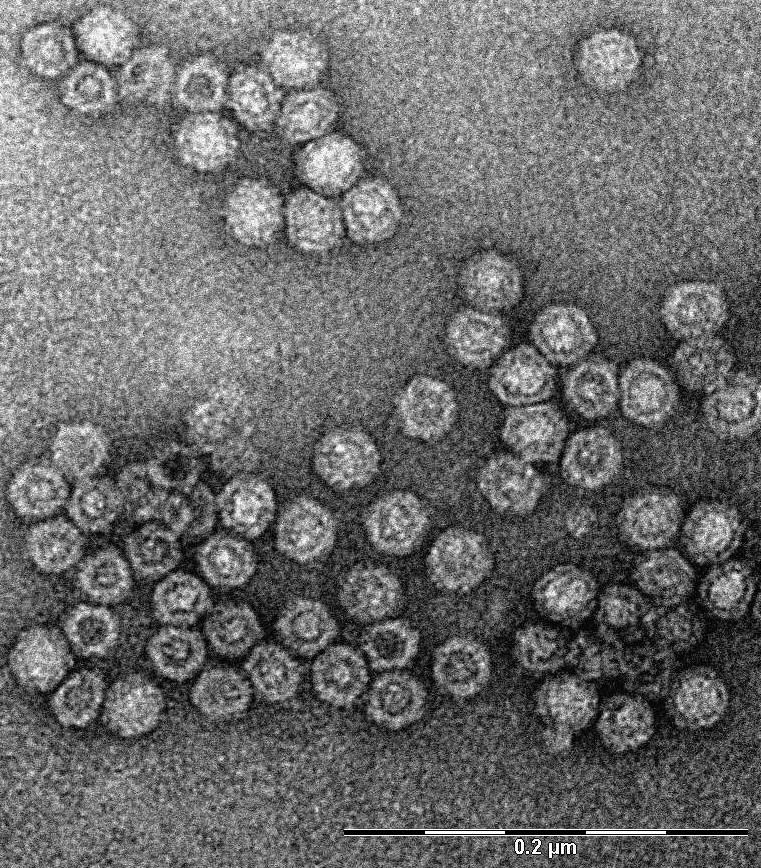
Transmission electron microscope image of virus-like particles. Magnification 140,000 times. Photo by Rasa Petraitytė-Burneikienė
Virus-like particles can be used to diagnose infectious diseases, vaccines, and production of preparations. Let's start from the beginning - what diseases are we talking about? Why are current diagnostic methods (such as PCR) inadequate?
Yes, virus-like particles can be used for influenza, hepatitis B, C, E, human papillomavirus, measles, rubella, and other disease diagnostics. The type of virus, stage of infection, and purpose of the examination determine which diagnostic methods to use.
PCR tests are designed to detect the genetic material of the virus: DNA or RNA. PCR tests are very specific and sensitive and they can detect very small amounts of the virus. Serological tests detect antibodies (IgM, IgG) produced by the immune system in response to a viral infection using viral proteins or virus-like particles. The PCR test is used to detect the virus at the beginning of the infection, in the early stages of the disease, as it detects the presence of the virus itself, which indicates that the virus is currently multiplying in the body. Serological testing is more accurate at a later stage of infection, usually after more than 10–14 days after the onset of infection, when the immune response begins to form and antibodies are produced. When serological tests are used at an early stage of infection, antibodies may not be detected, and the test will be negative. Serological tests are simpler, faster, and can be used for extensive epidemiological and population studies, it is possible to determine the spread of the virus and estimate the proportion of people with immunity to a certain virus.
If the virus is rapidly mutating, its genetic material can change a lot, and the PCR method will not detect the changed variant of the virus; then, the serological test becomes more reliable because it can detect a broader range of virus variants.
What would be the advantages of vaccines based on virus-like particles?
Virus-like particles are used to develop vaccines against viral or bacterial infections. Licensed vaccines - hepatitis B and human papillomavirus vaccines - have already been developed based on these particles.
The virus-like particles in the vaccine preparation present the human immune system with harmless virus-like structures resembling real viruses' outer envelope. These particles do not contain genetic material, so they cannot reproduce and cause disease or infection. However, they still trigger a robust immune response, which leads to the production of antibodies and memory cells that protect against infection.
Vaccines based on virus-like particles are safe because they do not contain live or inactivated viruses. There are no side reactions when using them. In addition, they are very effective vaccines because virus-like particles are usually very immunogenic, producing a robust immune response against them.
Virus-like particles can be engineered to have several different viral proteins on their surface, ensuring protection against multiple virus variants. This could help protect against rapidly mutating viruses such as influenza or HIV.
Which research results made you the happiest and motivated you to continue your work?
It would not be easy to single out one specific research, but I am motivated by the fact that my research is related to medicine. As a schoolgirl, I dreamed of becoming a doctor, but in the end, I chose the field of biochemistry. I have started working in a field where our research is significant in medicine. I am happy that I can contribute to improving human disease diagnostics.
Thanks for the answers!
Interviewed by Goda Raibytė-Aleksa